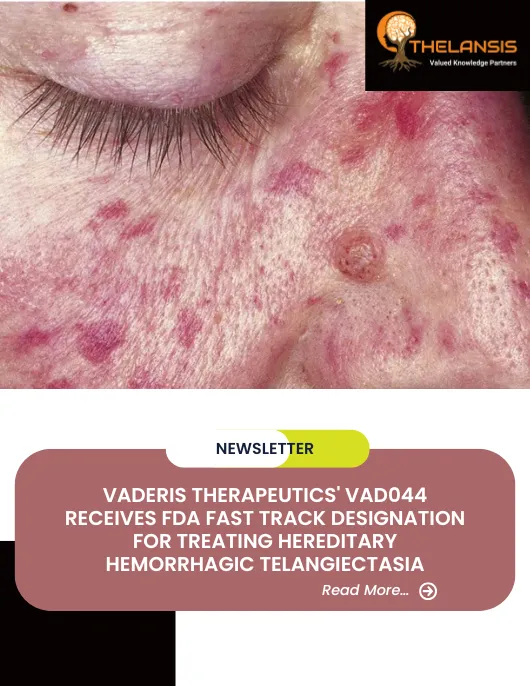
Vaderis Therapeutics’ VAD044 Receives FDA Fast Track Designation for Treating Hereditary Hemorrhagic Telangiectasia
Vaderis Therapeutics' VAD044 Receives FDA Fast Track Designation for Treating Hereditary Hemorrhagic Telangiectasia Vaderis Therapeutics AG, a clinical-stage biotechnology company focusing on treatmen ...
Vir Biotechnology Secures EMA Orphan Drug Designation for Tobevibart and Elebsiran in Treating Chronic Hepatitis Delta
Vir Biotechnology Secures EMA Orphan Drug Designation for Tobevibart and Elebsiran in Treating Chronic Hepatitis Delta Vir Biotechnology, Inc. has announced a significant milestone in the fight agains ...

