
FDA Clears CytoDyn’s Leronlimab for Phase II Trial in Colorectal Cancer
FDA Clears CytoDyn’s Leronlimab for Phase II Trial in Colorectal Cancer CytoDyn Inc., a biotechnology company specializing in the development of leronlimab, a CCR5 antagonist with potential for multip ...
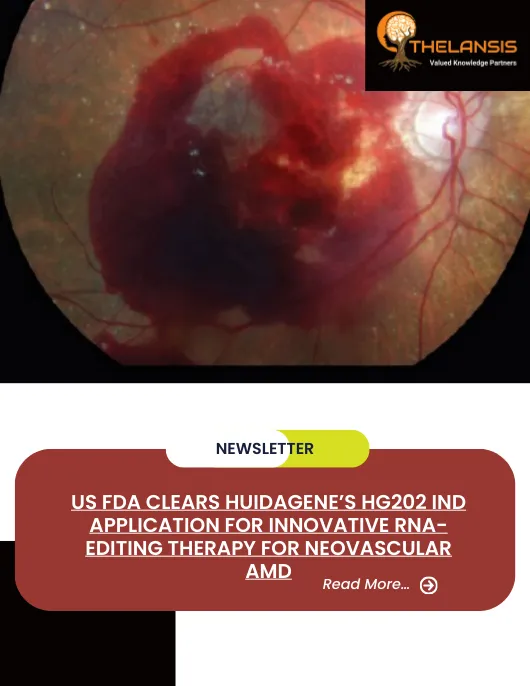
US FDA Clears HuidaGene’s HG202 IND Application for Innovative RNA-Editing Therapy for Neovascular AMD
HuidaGene Therapeutics (HuidaGene), a global clinical-stage biotechnology company specializing in genome medicines, has announced that the US FDA has cleared its HG202 investigational new drug (IND) a ...

