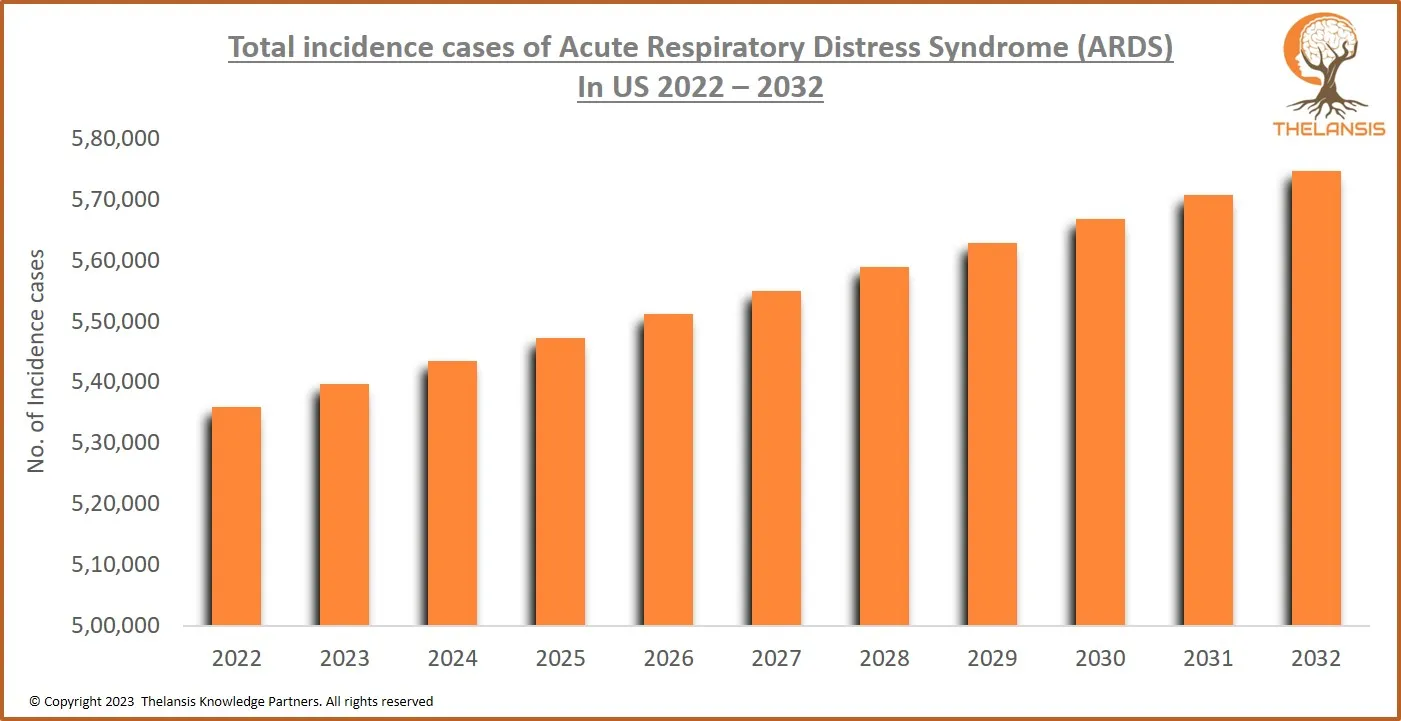
Total Incidence Cases of Acute Respiratory Distress Syndrome (ARDS) in U.S. 2022 – 2032
[vc_row][vc_column][vc_custom_heading text="Total Incidence Cases of Acute Respiratory Distress Syndrome (ARDS) in U.S. 2022 – 2032" font_container="tag:h2|text_align:center" use_theme_fonts="yes"][/v ...

