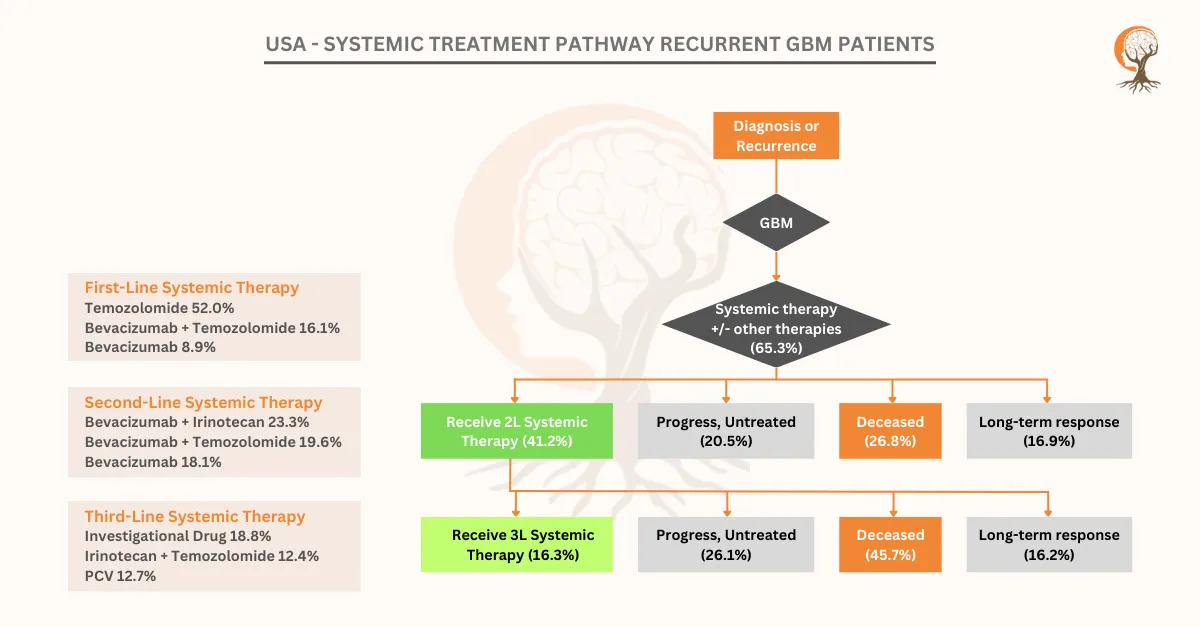
USA – Systemic Treatment Pathway Recurrent GBM Patients
[vc_row][vc_column][vc_custom_heading text="USA - Systemic Treatment Pathway Recurrent GBM Patients" font_container="tag:h2|text_align:center" use_theme_fonts="yes"][/vc_column][/vc_row][vc_row][vc_co ...

