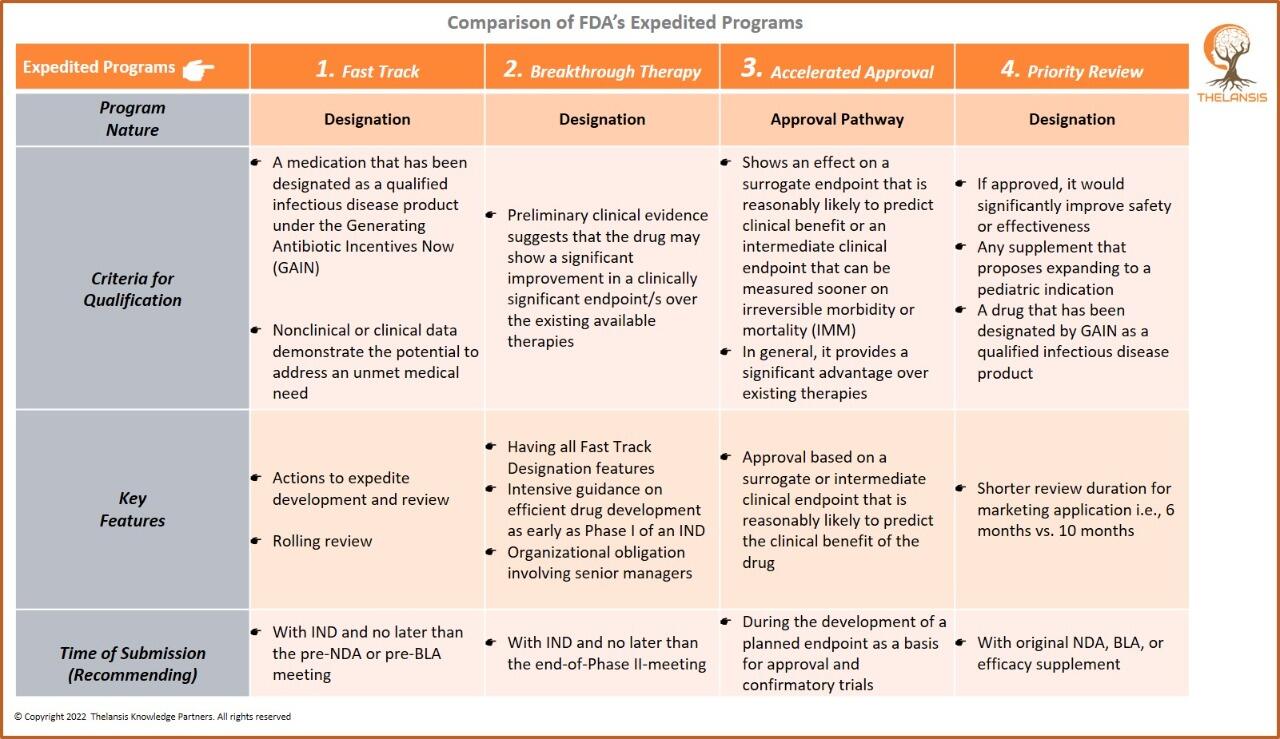
FDA Grants Fast-Track Designation to Tamibarotene for Higher-Risk Myelodysplastic Syndrome Treatment
Syros Pharmaceuticals, Inc., a biopharmaceutical company focused on improving treatment for hematologic malignancies patients through innovative standards of care, announces United States FDA has gran ...
Poxel SA Receives Orphan Drug Designation from the European Commission for PXL770 and PXL065 for Treatment of Adrenoleukodystrophy
POXEL SA, a biotech company developing treatments for serious diseases, announces the European Commission grant of orphan drug designation for PXL770 and PXL065 for Adrenoleukodystrophy treatment. The ...

DualityBio’s DB-1303 Granted Fast-Track Status by FDA for Advanced Endometrial Cancer Treatment
Duality Biologics has announced that the FDA has granted Fast Track Designation to DB-1303, a novel antibody-drug conjugate, for the treatment of advanced, recurrent or metastatic endometrial carcinom ...